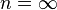Hi everyone today I am going to share Inplant training Experience in Ashok Leyland Company at ennore,Ennore plant is mainly for the Assembly purpose.
1)FRAME
ASSEMBLY:
- It is also the part of the chassis and also the supporting member of the vehicle.
- Mostly frame is assembled by using low carbon steel , because it should withstand some effects during the accident.If it is a brittle material , it will break when the heavy load or force is applied.
- Types of vehicle:
- Light commercial vehicle
- Heavy commercial vehicle
- Passenger type vehicle
- Multi acting vehicle
Depend
on the types frame will be designed.
- There will be several bracket is attached in the frame for several purpose like attach leaf springs,engine mounting etc.
- Front underrun protection device bracket is also used in frame assembly for safety purpose.
- Several cross member also fixed to withstand load.
- For multi acting vehicle,flanges will be provided to withstand heavy load.
- By using several instrument frame is assembled.
2)
Chassis Assembly:
- After finishing the frame assembly , chassis assembly is started.
- First of all , rear axle is fitted along air distributor and shake up jaw.
- Then front axle is fitted at the front along with power steering arrangement.
- The exhaust pipe and propeller shaft also connected.
- Propeller shaft is connected by universal joint , because this joint can motion in any direction.
- The engine is placed in the front side at the U-type cross member.It consists of piston,cylinder,fuel pump,two compressor.
- One compressor is used for pressurizing the air,another one is used for pressurizing the oil used for hydraulic forces in steering arrangement
- Along with alternator,fan for radiator is connected by belt,it is automatically run when the engine started and the whole arrangement run by this belt.
- Atlast top portion is fitted.
3)
Front axle assembly:
- Axle is mainly used to carry the weight and it is made by forged steel.
- Axle is bow shaped beam.The axle arm is fitted at the both the end.
- The axle arm is fixed by king pin.
- Then the brake drum is fixed.It consists of hub.brake liner,booster for brake,S-Cam.
- In Axle arm,the tie rod is connected between two wheels.It ensure that the both wheel turns in same direction.
4)Rear
axle Assembly:
- It is slightly different from front axle assembly.
- In this additionally differential arrangement is placed for rear wheel drive.
- First of all,hub is attached after that break drum arrangement is fixed.
- After that two bearing is fixed inside the brake drum arrangement for the fine rolling action.
- Then the half shaft is placed inside the differential arrangement at the both end.
5)Engine
assembly:
- In engine,engine case and flywheel is made by cast iron,piston is made by aluminium alloys,crank and cam is made by forged steel.
- The piston is placed in the engine case,the piston is connected to crank shaft by connecting rod.The small end of connecting rod is connected to piston and big end is connected to crank shaft.
- The fuel injector is placed above the cylinder.
- The cam is connected to crankshaft for the valve mechanism,the cam converts rotational motion into reciprocating motion for valve.
- For fuel injecting purpose,fuel pump is used,it transfer the fuel into the fuel injector.
- Turbo charger is fixed at the exhaust,another end is connected to inlet.It will increase the efficiency by transfer the inlet air quickly using compressor.
- Atlast alternator and fan for radiator is connected by belt.













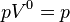
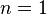
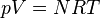
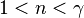

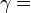
 is the isentropic exponent, yielding an isentropic process (adiabatic and reversible). It is also widely referred as adiabatic index, yielding an adiabatic process (no
is the isentropic exponent, yielding an isentropic process (adiabatic and reversible). It is also widely referred as adiabatic index, yielding an adiabatic process (no