Here we going to discuss about the some of the Thermodynamic process.First lets discuss about the what is thermodynamic process.
A thermodynamic process is a passage of a thermodynamic system from an initial state to a final state.In general, in a thermodynamic process, the system passes through physical states which are not describable as thermodynamic states, because they are far from internal thermodynamic equilibrium.
A thermodynamic process is a passage of a thermodynamic system from an initial state to a final state.In general, in a thermodynamic process, the system passes through physical states which are not describable as thermodynamic states, because they are far from internal thermodynamic equilibrium.
Isobaric Process:
An isobaric process occurs at constant pressure. An example would be to have a movable piston in a cylinder, so that the pressure inside the cylinder is always at atmospheric pressure, although it is
isolated from the atmosphere.From diagram we can see that from A to B pressure remains constant.
Isochoric Process:
An isochoric process is one in which the volume is held constant, meaning that the work done by the system will be zero. It follows that, for the simple system of two dimensions, any heat energy transferred to the system externally will be absorbed as internal energy. An isochoric process is also known as an isometric process or an isovolumetric process. An example would be to place a closed tin can containing only air into a fire. To a first approximation, the can will not expand, and the only change will be that the gas gains internal energy.From diagram we can see that from A to B Volume is constant
Isothermal process:
An isothermal process occurs at a constant temperature. An example would be to have a system immersed in a large constant-temperature bath. Any work energy performed by the system will be lost to the bath, but its temperature will remain constant.
Adiabatic Process:
An adiabatic process is a process in which there is no energy added or subtracted from the system by heating or cooling. For a reversible process, this is identical to an isentropic process. The system is thermally insulated from its environment and that its boundary is a thermal insulator. If a system has an entropy which has not yet reached its maximum equilibrium value, the entropy will increase even though the system is thermally insulated. Under certain conditions two states of a system may be considered adiabatically accessible
Isentropic Process:
An isentropic process occurs at a constant entropy. For a reversible process this is identical to an adiabatic process. If a system has an entropy which has not yet reached its maximum equilibrium value, a process of cooling may be required to maintain that value of entropy.
Polytropic process:
A polytropic process is a thermodynamic process that obeys the relation:
where P is the pressure, V is volume, n is any polytropic index, and C is a constant. This equation can be used to accurately characterize processes of certain systems, notably the compression or expansion of a gas, but in some cases, liquids and solids.
isentropic process and polytropic process difference:
1. isentropic is reversible adiabatic process.polytropic process is one of the proces of thermodynamic.
2. isentropic perform![[IMG]](https://lh3.googleusercontent.com/blogger_img_proxy/AEn0k_tuX1rI31xPNRGQTth4Fj4WQQgl429Qlo3ac5Lonn7p-g31mFUvFZP7fo2JcXBe81QuThTOxyZkKjId1M7yiLSn3QJ58gMQjYIBKRRjDzYlNk8hIIMw46P9nhjZ0WNojwLZSMuHngsaI74s92Ofnw=s0-d) . but polytropic perform
. but polytropic perform ![[IMG]](https://lh3.googleusercontent.com/blogger_img_proxy/AEn0k_vrA8qhjrCJtHoy3sMq7yqUmxii_w-hIAj4dkq4JRRLoiFshaXaRl370KAsfQaImgpIj_5M6ZVeV0hgWZZd4tvpBgcgpuiaSMHdiGbpn6kcU_gFeyMZZ4EmNJEZAC7gtn6itNX5mLuPAQIckGiQ6Q=s0-d) ,pv reletion. here p is pressure in pascal. v= volume meret cube. and y=adiabatic index,and n= polytropic index.
,pv reletion. here p is pressure in pascal. v= volume meret cube. and y=adiabatic index,and n= polytropic index.
3. isentropic process only work transfer no heat transfer. but in polytropic heat and work both can be transfer.
4.polytropic index "n" always less then adiabatic index "y",but greater then 1.
1. isentropic is reversible adiabatic process.polytropic process is one of the proces of thermodynamic.
2. isentropic perform
3. isentropic process only work transfer no heat transfer. but in polytropic heat and work both can be transfer.
4.polytropic index "n" always less then adiabatic index "y",but greater then 1.
Relationship to ideal processes[edit]
For certain values of the polytropic index, the process will be synonymous with other common processes. Some examples of the effects of varying index values are given in the table.
| Polytropic index | Relation | Effects |
|---|---|---|
 | — | Negative exponents reflect a process where the amount of heat being added is large compared to the amount of work being done (i.e. the energy transfer ratio > γ/(γ-1)). Negative exponents can also be meaningful in some special cases not dominated by thermal interactions, such as in the processes of certain plasmas in astrophysics. |
 | 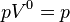 (constant) | Equivalent to an isobaric process (constant pressure) |
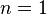 | 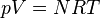 (constant) | Equivalent to an isothermal process (constant temperature) |
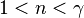 | — | A quasi-adiabatic process in which the heat flow and work flow are in opposite directions (positive K), such as in vapor compression refrigeration during compression, where the elevated vapour temperature resulting from the work done by the compressor on the vapour leads to some heat loss from the vapour to the cooler surroundings. Also a "polytropic compression" process like gas through a centrifugal compressor where heat loss from the compressor (into environment) is greater than the heat added to the gas through compression. |
 | — | 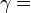  is the isentropic exponent, yielding an isentropic process (adiabatic and reversible). It is also widely referred as adiabatic index, yielding an adiabatic process (no heat transferred). However the term adiabatic does not adequately describe this process, since it only implies no heat transfer.[3] Only a reversible adiabatic process is an isentropic process. is the isentropic exponent, yielding an isentropic process (adiabatic and reversible). It is also widely referred as adiabatic index, yielding an adiabatic process (no heat transferred). However the term adiabatic does not adequately describe this process, since it only implies no heat transfer.[3] Only a reversible adiabatic process is an isentropic process. |
 | — | Normally polytropic index is greater than specific heat ratio (γ) within a "polytropic compression" process like gas through a centrifugal compressor. The inefficiencies of centrifugal compression and heat added to the gas outweigh the loss of heat into the environment. Also a quasi-adiabatic process in which the heat flow and work flow are in the same direction (negative K), such as in an internal combustion engine during the power stroke, where heat is lost from the hot combustion products, through the cylinder walls, to the cooler surroundings, at the same time as those hot combustion products do work on the piston. |
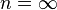 | — | Equivalent to an isochoric process (constant volume) |
Quasistatic process:
A quasistatic process is a thermodynamic process that happens slow enough for the system to remain in internal equilibrium. An example of this is quasistatic compression, where the volume of a system changes at a rate slow enough to allow the pressure to remain uniform and constant through out the system








